Enhanced Lithium–Sulfur Battery Performance Raises Hopes for Eco-Friendly Batteries
- Input
- 2026-01-09 08:31:00
- Updated
- 2026-01-09 08:31:00
[Financial News] A new technology has been developed that simultaneously boosts the performance and lifespan of the lithium–sulfur battery. Experts say it could pave the way for next-generation eco-friendly batteries for Electric Vehicles (EV), drones, and large-scale energy storage system (ESS) applications.
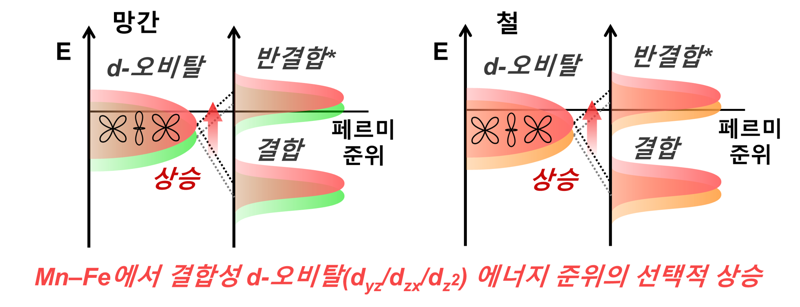
A research team led by Professor Kim Won Bae from the Department of Chemical Engineering and the Department of Battery Engineering at Pohang University of Science and Technology (POSTECH), together with master’s student Sangyeon Won from the Department of Battery Engineering and integrated Ph.D. student Joonhyuk Ji from the Department of Chemical Engineering, announced on the 9th that they have successfully designed a bimetallic catalyst in which manganese (Mn) and iron (Fe) atoms are bonded. This catalyst accelerates reaction kinetics while maintaining stability. The study was published in the Journal of Energy Chemistry, an international journal in the fields of energy and materials chemistry.
The lithium–sulfur battery can theoretically store more energy than a conventional Lithium-ion battery (Li-ion battery), and it uses inexpensive, lightweight sulfur, making it ideal for applications that require lightweight, high-energy batteries such as drones and urban air mobility (UAM). However, lithium polysulfide (LiPSs) generated during charge and discharge migrates within the cell and causes the so-called shuttle effect, which drains energy and leads to reduced battery life and efficiency.
To address this, the team applied a more advanced concept than the existing single-atom catalyst, namely a dual-atom catalyst (DAC). With two metal atoms positioned close to each other and interacting, the catalyst can strongly anchor sulfur species inside the battery while accelerating the electrochemical reactions.
The researchers synthesized a DAC composed of manganese and iron and used computer simulations to confirm that the electronic structure changes selectively when the two metals are bonded. Thanks to these changes, the catalyst can firmly bind lithium polysulfide while promoting its rapid conversion, which speeds up the battery reactions and reduces the loss of intermediate species.
They also focused on improving the stability of the lithium metal anode. In conventional systems, lithium does not deposit uniformly during charging, causing a rough surface and shortening battery life. When the DAC was introduced, lithium was deposited evenly, forming a stable metallic surface. In practical tests, the cell maintained its initial capacity and showed stable performance even after hundreds of charge–discharge cycles.
Professor Kim Won Bae stated, “By revealing how the electronic structure between metals changes at the atomic level, we have demonstrated a principle that can simultaneously enhance reaction speed and stability in batteries.” He added, “This DAC design strategy will open a new pathway for developing next-generation high–energy-density batteries.”
jiany@fnnews.com Yeon Ji-an Reporter