Obesity Drug Development in Full Swing: 'Improving Convenience and Efficacy, Reducing Side Effects'
- Input
- 2025-10-27 15:34:28
- Updated
- 2025-10-27 15:34:28

[Financial News] As GLP-1 class anti-obesity drugs have emerged as game-changers in the global pharmaceutical and biotech market, the treatment paradigm, which has been centered on injectables, is rapidly shifting. Companies are diversifying formulations to improve convenience and efficacy, with a strong focus on minimizing the burden of administration.
According to the pharmaceutical and biotech industry on the 27th, GLP-1 class anti-obesity drugs work by lowering blood sugar and increasing satiety to promote weight loss. Notable examples include Wegovy (active ingredient: Semaglutide) and Mounjaro (tirzepatide).
These injectable drugs have demonstrated impressive weight loss effects of 15–20% with just one weekly dose, driving explosive demand. However, factors such as aversion to injections, skin irritation, and the need for refrigeration have negatively impacted patient adherence.
As a result, global pharmaceutical companies are now competing on two fronts: maintaining efficacy and improving ease of administration. They are diversifying formulations to reduce side effects and minimize the burden of taking the medication.
Eli Lilly and Company, which has taken the lead in the GLP-1 market with Mounjaro, is ushering in a new era with its oral anti-obesity drug, Orforglipron.
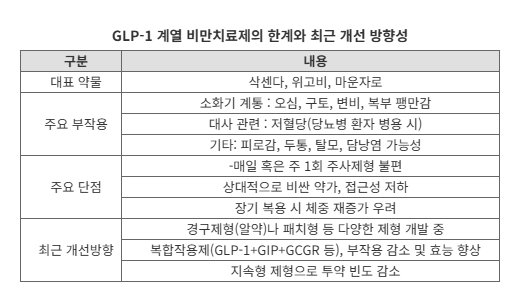
This medication, formulated as a pill for stable absorption in the body, is also reported to have a lower incidence of gastrointestinal side effects such as nausea and vomiting. As a result, it is praised for combining both dosing convenience and tolerability.
In Korea, Ildong Pharmaceutical has shown promise with meaningful results from clinical trials of an oral anti-obesity drug with a similar mechanism. Hanmi Pharmaceutical recently exported its platform technology, which converts injectable drugs into oral formulations, to Gilead Sciences in the United States, demonstrating the competitiveness of next-generation drug delivery technologies.
Research is expanding beyond injections and pills to include formulations that can be applied to the skin or implanted subcutaneously. In Korea, Daewoong Pharmaceutical received approval from the Ministry of Food and Drug Safety (MFDS) for a phase 1 clinical trial of a microneedle patch-type anti-obesity drug using Semaglutide.
Nevertheless, the side effects of GLP-1 class drugs remain a challenge to overcome. The most common side effects are gastrointestinal symptoms such as nausea, vomiting, abdominal bloating, constipation, and diarrhea.
Some patients experience discomfort from appetite loss, fatigue, or gallstone formation, rather than weight loss. Experts point out that these side effects often stem from abrupt changes in drug concentration or failure to adjust the dosage properly.
According to current clinical data, it is difficult to completely eliminate the side effects of GLP-1 class drugs. However, it is considered feasible to reduce their frequency and severity by controlling drug release rates, introducing combination mechanisms, and diversifying formulations.
An industry official explained, "The obesity drug market is moving beyond simple 'weight loss competition' to focus on how to achieve safer and more convenient weight reduction." The official added, "If the surge of GLP-1 class injectables has transformed the paradigm of obesity treatment, now pills, patches, and implantable formulations are expanding the boundaries of innovation."
vrdw88@fnnews.com Kang Jung-mo Reporter