"There is an edible Wegovy"... Influencers deceive consumers, selling 32.4 billion won
- Input
- 2025-08-20 14:35:17
- Updated
- 2025-08-20 14:35:17
Food and Drug Safety Administration, sends 5 companies to prosecution for false and exaggerated advertising
Violations of the law by riding the domestic and international craze for obesity treatments
Ordinary foods cannot achieve the efficacy of pharmaceuticals
Violations of the law by riding the domestic and international craze for obesity treatments
Ordinary foods cannot achieve the efficacy of pharmaceuticals
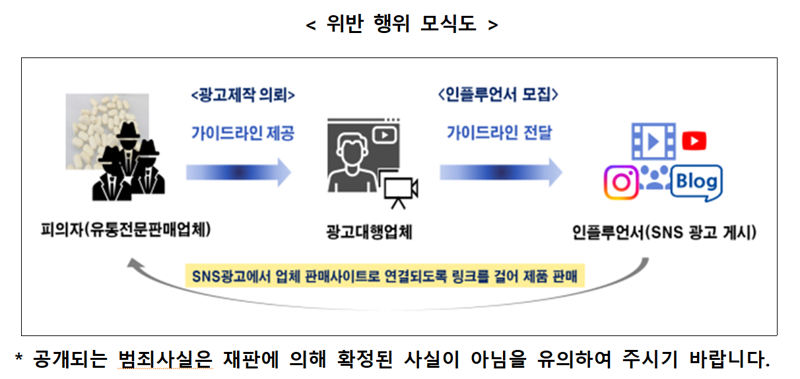
[Financial News] Companies that disguised ordinary food as 'edible Wegovy' by riding the craze for obesity treatments and sold 32.4 billion won worth of products have been caught. The Food and Drug Safety Administration announced on the 20th that it has sent five company representatives to prosecution for violating the Food Labeling and Advertising Act by engaging in false and exaggerated advertising through SNS such as YouTube and Instagram.
According to the Food and Drug Safety Administration, these companies packaged simple ordinary foods such as processed fruits and vegetables, beverage bases, and similar processed products as health functional foods or pharmaceuticals and sold them.
Company A advertised processed fruits and vegetables and solid tea products with phrases like 'body fat reduction' and 'fat removal', selling the most among single companies with 25.5 billion won worth. Company B promoted its beverage base product as 'the same principle as Wegovy' and sold about 5.1 billion won, while Company D used expressions like 'effective for diet and weight loss' to sell similar processed products, achieving sales of 1.5 billion won.
Companies C and E also recorded sales of several hundred million won by using pharmaceutical-like expressions such as 'appetite suppressant' or 'GLP-1 principle'.
They deceived consumers with advertisements in the form of reviews by influencers. Influencers packaged keywords provided by the company, such as '7kg weight loss in one month' and 'ultra-powerful appetite suppressant', as their own experiences, posting videos and writings, with direct links to purchase sites included in the posts.
Ultimately, consumers believed these to be personal experience reviews and purchased the products, but in reality, it was 'manipulated advertising' planned by the company.
This incident shows the shadow of the rapidly growing diet market following the recent release of obesity treatments. In this atmosphere, expressions like 'edible Wegovy' became rampant, and situations arose where ordinary foods were disguised as pharmaceuticals.
With new GLP-1 class drugs like 'Wegovy' and 'Mounjaro' gaining worldwide attention, domestic consumers are also looking for easy alternatives to lose weight.
However, any advertising phrases that guarantee weight loss effects in a short period should be doubted first. Ordinary foods cannot achieve the efficacy of pharmaceuticals.
The problem is the institutional loophole. It is legal for consumers to post personal experience reviews, but it is illegal for businesses to post manipulated reviews for commercial purposes. However, due to the nature of SNS, the boundary between advertising and personal experiences is ambiguous, making it difficult to regulate, and it is easy to lead to the actual purchase stage.
Consumers end up having excessive expectations that ordinary foods will have effects like new drugs, inevitably leading to harm.
The Food and Drug Safety Administration plans to strengthen the crackdown on illegal online advertising in the future and urges consumers to check the functionality recognition status on the 'Food Safety Korea' website when purchasing health functional foods.
vrdw88@fnnews.com Kang Jung-mo Reporter