Wells Bio Obtains European Certification for 8 Types of Molecular Diagnostic Reagents for Infectious Disease Diagnosis
- Input
- 2025-08-20 10:15:08
- Updated
- 2025-08-20 10:15:08
CE-IVDR certification according to EU In Vitro Diagnostic Medical Device Regulation
6 types of diagnostic reagents for sexually transmitted infectious diseases and 2 types of diagnostic reagents for tick-borne infectious diseases
6 types of diagnostic reagents for sexually transmitted infectious diseases and 2 types of diagnostic reagents for tick-borne infectious diseases
[Financial News] Wells Bio announced on the 20th that it has received the 'CE-IVDR' conformity certification according to the European Union (EU) In Vitro Diagnostic Medical Device Regulation for 8 types of multiple molecular diagnostic reagents for infectious disease diagnosis.
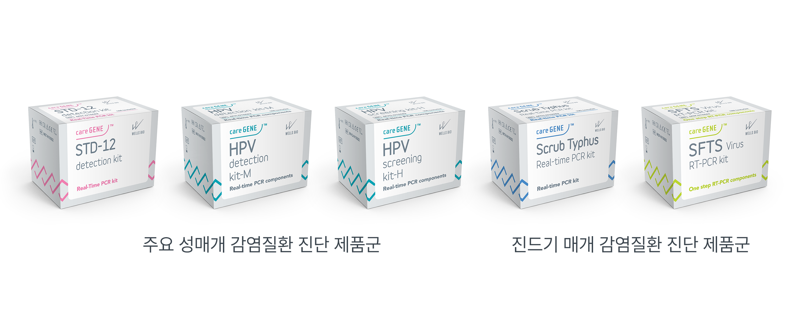
The multiple molecular diagnostic reagents for infectious disease diagnosis that obtained this certification include 6 types of diagnostic reagents for sexually transmitted infectious diseases and 2 types of diagnostic reagents for tick-borne infectious diseases.
The certified products are △ 'Caregene STD12 Detection Kit' which can simultaneously detect 12 types of major sexually transmitted disease pathogens △ 'Caregene HPV Screening Kit H' which can screen 14 types of human papillomavirus (HPV) △ 'Caregene HPV Detection Kit M' which can screen 7 high-risk and 18 low-risk HPV types △ 'Caregene Scrub Typhus Real-time PCR Kit' for diagnosing scrub typhus and severe fever with thrombocytopenia syndrome (SFTS).
Lee Minjeon, CEO of Wells Bio, said, "It proves that the excellence of multiple molecular diagnostic technology and infectious disease diagnostic reagents meets global standards," and added, "We will continue to expand our market share and strengthen our competitiveness in the global market, including Europe, through continuous research and development and technology advancement."
kaya@fnnews.com Choi Hyerim Reporter