GI Innovation Allergy Treatment GI-301 Receives Substance Patent in the US
- Input
- 2025-07-28 13:37:22
- Updated
- 2025-07-28 13:37:22
Patent Protection for GI-301's Composition and Quality Intellectual Property
[Financial News] GI Innovation announced on the 28th that a substance patent based on the structural combination of proteins for the next-generation allergy treatment GI-301 (ingredient name Resigercept · development code YH35324) has been registered in the United States. This patent secures rights to the main ingredient of GI-301 itself and completes an intellectual property system that can doubly protect GI-301 in terms of both composition and quality, along with the sialic acid high-content-based patent registered in the US last May.
The previously registered sialic acid high-content patent is a quality-centered patent based on the fact that if the sugar component 'sialic acid' on the protein surface exists at a high level, it is advantageous for the development of subcutaneous injection (SC) formulations due to extended half-life and improved drug stability.
In fact, GI-301 is currently being developed as an SC formulation, and the sialic acid high-content patent supports this formulation competitiveness patent-wise.
On the other hand, the newly registered patent protects the unique amino acid sequence and protein structure of GI-301, securing the most basic and essential rights to the ingredient itself.
The two patents act complementarily, forming a solid patent portfolio that supports the technical differentiation and development competitiveness of GI-301.
The US Patent Office recognized the progressiveness of GI-301, noting its demonstrated unpredictable level of biological activity, and particularly mentioned its excellent IgE inhibition efficacy in the mechanism of allergic reactions as a major evaluation factor.
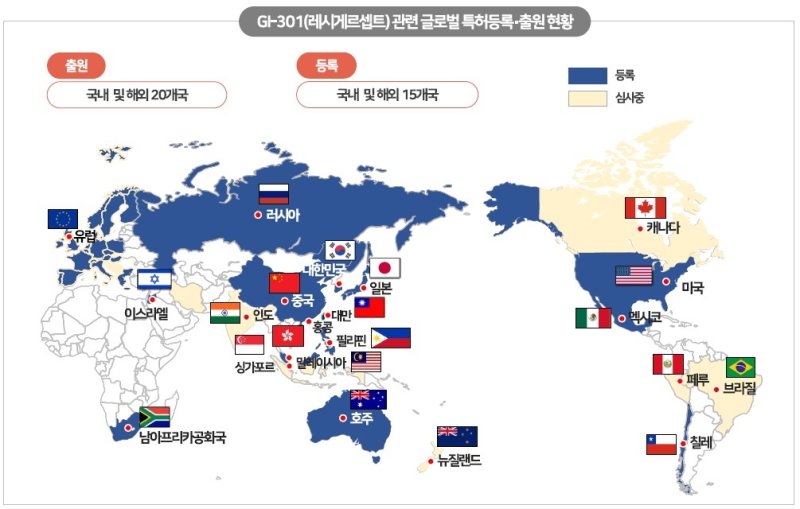
GI-301-related patents have currently been filed in a total of 21 countries, and registration has been completed in 16 major countries, including the US, Europe, China, and Japan, effectively completing the establishment of a rights network in the global market.
According to clinical results presented by Yuhan Corporation at the European Academy of Allergy and Clinical Immunology (EAACI) last June, GI-301 showed a stronger and more sustained free IgE inhibitory effect compared to the existing treatment Xolair in a small-scale phase 1b trial targeting patients with chronic spontaneous urticaria (CSU), and the complete remission patient ratio was also excellent based on the representative evaluation index UAS7 (7-day average urticaria score).
Jang Myung-ho, CEO of GI Innovation, said, "A new drug with global competitiveness is completed not only with excellent clinical data but also with strong intellectual property protection," adding, "The essential ingredient protection patent secured this time and the existing sialic acid-based quality patent act complementarily, further strengthening the structural and quality competitiveness of GI-301."
He added, "It will also be an important foundation for future joint development and global technology transfer with Yuhan Corporation."
vrdw88@fnnews.com Kang Jung-mo Reporter